How Water Works?
In its purest form, it's odorless, nearly colorless and tasteless. It's in your body, the food you eat and the beverages you drink. You use it to clean yourself, your clothes, your dishes, your car and everything else around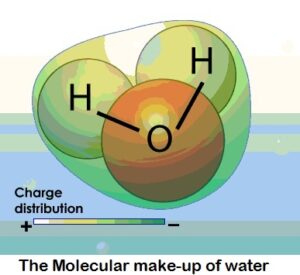 you. You can travel on it or jump in it to cool off on hot summer days. Many of the products that you use every day contain it or were manufactured using it. All forms of life need it, and if they don't get enough of it, they die. Political disputes have centered around it. In some places, it's treasured and incredibly difficult to get. In others, it's incredibly easy to get and then squandered. What substance is more necessary to our existence than any other? Water.
you. You can travel on it or jump in it to cool off on hot summer days. Many of the products that you use every day contain it or were manufactured using it. All forms of life need it, and if they don't get enough of it, they die. Political disputes have centered around it. In some places, it's treasured and incredibly difficult to get. In others, it's incredibly easy to get and then squandered. What substance is more necessary to our existence than any other? Water.
At its most basic, water is a molecule with one oxygen atom and two hydrogen atoms, bonded together by shared electrons. It is a V-shaped polar molecule, which means that it's charged positively near the hydrogen atoms and negatively near the oxygen atom. Water molecules are naturally attracted and stick to each other because of this polarity, forming a hydrogen bond. This hydrogen bond is the reason behind many of water's special properties, such as the fact that it's denser in its liquid state than in its solid state (ice floats on water).
Water is the only substance that occurs naturally as a solid (ice), a liquid and a gas (water vapor). It covers about 70 percent of the Earth for a total of approximately 332.5 million cubic miles (1,386 million cubic kilometers). If you're familiar with the lines "Water, water, everywhere, nor any drop to drink" from the poem "The Rime of the Ancient Mariner," you'll understand that most of this water -- 97 percent of it -- is undrinkable because it's saltwater. Only 3 percent of the world's water supply is freshwater, and 77 percent of that is frozen. Of the 23 percent that is not frozen, only a half a percent is available to supply every plant, animal and person on Earth with all the water they need to survive.
So water is pretty simple, right? Actually, there are a lot of things about it that scientists still don't fully understand. And the problem of making sure that enough clean, drinkable water is available to everyone and everything that needs it is anything but simple. In this article, we'll look at some of these problems. We'll also explore exactly what plants, animals and people do with water and learn more about what makes water so special.
The World's Water Supply
There's often discussion in the news of the world's dwindling water supply, but this isn't entirely accurate. The amount of water isn't diminishing, but the demand for it is steadily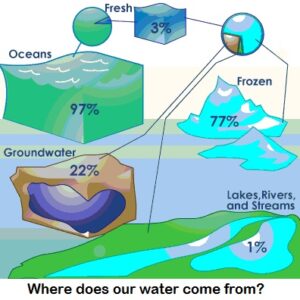 increasing. Some scientists believe that the world's population, currently at 6 billion, will double by 2050. In addition, the amount of water that is clean and drinkable is steadily decreasing because of pollution.
increasing. Some scientists believe that the world's population, currently at 6 billion, will double by 2050. In addition, the amount of water that is clean and drinkable is steadily decreasing because of pollution.
For many people in industrialized countries, getting water is as easy as turning on a faucet, and it's rather inexpensive. But freshwater isn't evenly distributed throughout the world. More than half of the world's water supply is contained in just nine countries: the United States, Canada, Colombia, Brazil, the Democratic Republic of Congo, Russia, India, China and Indonesia. Urban areas, obviously, have a greater need for water beyond the basics for drinking and sanitation. But overpopulation in undeveloped countries means that many people don't even get the basics.
Most of the world's freshwater -- about 2.4 million cubic miles (10 million cubic kilometers) of it is contained in underground aquifers. The rest comes from:
Rainfall (after accounting for evaporation): 28,500 cubic miles (119,000 cubic kilometers)
Man-made reservoirs: 1,200 cubic miles (5,000 cubic km)
Lakes: 21,830 cubic miles (91,000 cubic km)
Rivers: 509 cubic miles (2,120 cubic km)
Water distribution has everything to do with political boundaries, economic development and wealth. In Mexico City, for example, 9 percent of the population uses 75 percent of the available water, and a crumbling infrastructure means that up to half of the water supply is lost through pipe leaks and evaporation.
Some countries don't have enough clean water for their rapidly growing populations, and they can't afford the infrastructure necessary to clean and transport it. For example, most people in China's cities suffer from water shortages, and most of China's groundwater, lakes and rivers are polluted. About 700 million Chinese people have access only to drinking water that does not meet standards set by the World Health Organization.
Countries in the Middle East use the least amount of water per person because there are so few natural sources of freshwater. In contrast, the usage of water is higher in the United States than in any other country, with around 60,000 cubic feet (1,700 cubic meters) of water used per person in 2002. But even within the United States, there are some states and regions that don't contain enough water to supply their populations. Coastal regions of Florida have so much saltwater that they must have freshwater piped in from inland areas, which has led to political disputes over control of the water supply.
Water Regulation
In many areas, water is regulated and distributed by governments. In the United States, it's regulated by the Safe Drinking Water Act. However, government control isn't always in the best interests of all people. In the 1930s, to irrigate cotton fields, the Soviet government created canals to divert the rivers that fed the Aral Sea (located between Kazakhstan and Uzbekistan). As a result, the surface area of the sea has shrunk by more than 50 percent and its volume by 80 percent over the past 50 years. Its salinity increased and it became polluted with pesticides, fertilizer runoff and industrial waste. The loss of the sea meant the decline of the commercial fishing industry, which helped to send the region into poverty. The pollutants from the exposed seabed have been found in the blood of Antarctic penguins.
the best interests of all people. In the 1930s, to irrigate cotton fields, the Soviet government created canals to divert the rivers that fed the Aral Sea (located between Kazakhstan and Uzbekistan). As a result, the surface area of the sea has shrunk by more than 50 percent and its volume by 80 percent over the past 50 years. Its salinity increased and it became polluted with pesticides, fertilizer runoff and industrial waste. The loss of the sea meant the decline of the commercial fishing industry, which helped to send the region into poverty. The pollutants from the exposed seabed have been found in the blood of Antarctic penguins.
Some regions have privatized their water distribution, which has often led to conflict. In the late 1980s, the United Kingdom sold its water boards (governmental water-supply organizations) to private companies, which improved the infrastructure. Many people were outraged that companies could profit off such a basic need, especially when people who could not pay their bills suffered harsh penalties. The problem was later remedied with legislation.
In 2000 and 2005, demonstrators took to the streets in Bolivia to protest the privatization of the water supply. When foreign companies took over Bolivia's water system, the cost of water became too expensive for the poor. In the city of El Alto, "the cost of getting a water and sewage hook-up exceeded a half-year's income at the minimum wage". The 2000 revolt, called the "Bolivian Water Wars," led to martial law and 100 injuries. After both incidents, the Bolivian government canceled the private company contracts.
Currently, more than a billion people, about 17 percent of the world's population, don't have access to clean water. There are several governmental and nongovernmental organizations, including UNICEF and Water Aid, working to help poor communities in Asia and Africa obtain sustainable supplies of drinking water and sanitation facilities. Water shortages happen in the United States, too -- many states have programs to assist the disadvantaged with obtaining enough water and paying their water and sewer bills.
Human Water Consumption
Our bodies are about 60 percent water. Water regulates our body temperature, moves nutrients through our cells, keeps our mucous membranes moist and flushes waste from our 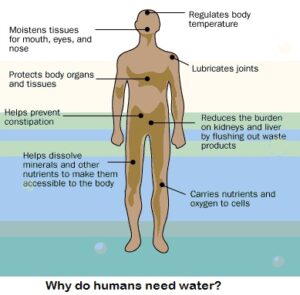 bodies. Our lungs are 90 percent water, our brains are 70 percent water and our blood is more than 80 percent water. Simply put, we can't function without it. Most people sweat out about two cups of water per day (0.5 liters). Each day, we also lose a little more than a cup of water (237 ml) when we exhale it, and we eliminate about six cups (1.4 l) of it. We also lose electrolytes -- minerals like sodium and potassium that regulate the body's fluids. So how do we replace it?
bodies. Our lungs are 90 percent water, our brains are 70 percent water and our blood is more than 80 percent water. Simply put, we can't function without it. Most people sweat out about two cups of water per day (0.5 liters). Each day, we also lose a little more than a cup of water (237 ml) when we exhale it, and we eliminate about six cups (1.4 l) of it. We also lose electrolytes -- minerals like sodium and potassium that regulate the body's fluids. So how do we replace it?
We can get about 20 percent of the water we need through the food we eat. Some foods, like watermelon, are nearly 100 percent water. Although the amount of water that we need each day varies, it's usually about eight cups (2 l). But instead of worrying about getting in those eight cups, you should just drink when you start to feel thirsty. You can get your water by drinking other beverages -- but some beverages, like alcohol, can make you more dehydrated.
If your urine is dark yellow, you might not be drinking enough water. Of course, you need more water when you're exercising; ill with diarrhea, vomiting or fever; or in a hot environment for a long time. Most people can survive only a few days without water, although it depends on a number of factors, including their health and environment. Some have gone as long as two weeks. Followers of a Buddhist boy meditating in Nepal claim that he has gone two years without food or water, but doctors have not been able to substantiate this.
When you don't get enough water, or lose too much water, you become dehydrated. Signs of mild dehydration include dry mouth, excessive thirst, dizziness, lightheadedness and weakness. If people don't get fluids at this point, they can experience severe dehydration, which can cause convulsions, rapid breathing, a weak pulse, loose skin and sunken eyes. Ultimately, dehydration can lead to heart failure and death.
Dehydration caused by diarrhea is a major cause of death in undeveloped countries. Nearly 2 million people, mostly children, die from it each year [source: WHO]. Consuming water polluted with biological contaminants and not having access to adequate sanitary facilities can lead to diseases like malaria and cholera and parasites like cryptosporidiosis and schistosomiasis. Water can be also be contaminated with chemicals, pesticides and other naturally occurring
Water Purification
Water that is safe to drink is called potable water, or drinking water, in contrast to safe water, which can be used for bathing or cleaning. In the United States, the Environmental Protection Agency sets maximum levels for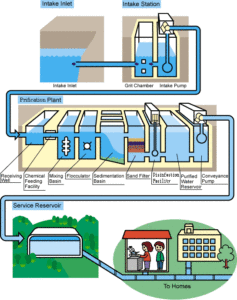 the 90 most commonly occurring contaminants. If something happens to your water supply, your supplier has to contact you to let you know what precautions you should take.
the 90 most commonly occurring contaminants. If something happens to your water supply, your supplier has to contact you to let you know what precautions you should take.
Water treatment requires six basic steps.
-
In coagulation, coagulants like lime and alum are added to the water, which causes particulates to clump together.
-
Next, the water is shaken into larger clumps, called flocs.
-
The sedimentation process requires that the water stand for 24 hours, which allows the clumps to settle to the bottom.
-
The water is then filtered, disinfected (usually with chlorine) and aerated.
-
Aeration helps to remove certain contaminants like radon.
Water Survival Techniques
If your water becomes contaminated and you don't have bottled water, you can purify it in a few different ways. If it is cloudy, first filter it through clean cloths or allow it to settle and then pour off the clear water. Then, you can boil the water for one minute to kill most disease-causing organisms.
You can also add one-eighth of a teaspoon of household chlorine bleach per gallon of water (or follow directions on the label). You should double the amount if the water is discolored or murky. Stir and let it stand for 30 minutes. Chlorine bleach tablets are sold at camping supply stores to purify water for drinking. You can also use five drops of iodine per gallon to disinfect water.
Store boiled or disinfected water in clean, covered containers. If the boiled water tastes too flat or the chlorine taste is too strong, pour it from one container into another.
The Water Cycle
The water cycle is the continuous movement of water in and around the Earth. As previously mentioned, water never really goes away -- it just changes form. The sun drives the entire water cycle and is responsible for its two major components: condensation and evaporation. When the sun heats the surface of water, it evaporates and ends up in the atmosphere as water vapor. It cools and rises, becoming clouds, which eventually condense into water droplets. Depending on the temperature of the atmosphere and other conditions, the water precipitates as rain, sleet, hail or snow.
entire water cycle and is responsible for its two major components: condensation and evaporation. When the sun heats the surface of water, it evaporates and ends up in the atmosphere as water vapor. It cools and rises, becoming clouds, which eventually condense into water droplets. Depending on the temperature of the atmosphere and other conditions, the water precipitates as rain, sleet, hail or snow.
Some of this precipitation is captured by tree canopies and evaporates again into the atmosphere. The precipitation that hits the ground becomes runoff, which can accumulate and freeze into snow caps or glaciers. It can also infiltrate the ground and accumulate, eventually storing in aquifers. An aquifer is a large deposit of groundwater that can be extracted and used. This runoff also comes from snowmelt, which occurs when the sun and climate changes melt snow and ice. Finally, some of this runoff makes it way back into lakes and oceans, where it is again evaporated by the sun. You can learn more about the water cycle in How the Earth Works.
Water that falls to the ground and stays in the soil ends up evaporating and retiring to the atmosphere. But groundwater, which is the major source of our drinking water, can accumulate in aquifers over thousands of years. Unconfined aquifers have the water table, or the surface where water pressure equals atmospheric pressure, as their upper boundaries. Confined aquifers often lie below unconfined aquifers and have a layer of rock or other materials as their upper boundaries.
In the United States, the oldest groundwater, known as fossil water, is contained in the Ogallala Aquifer. Lying below about 175,000 square miles (450,000 square kilometers) of eight states in the Great Plains, the Ogallala Aquifer stores about 2,900 million acre-feet (3,600 million kilometers cubed) of water [source: High Plains/Ogallala Aquifer]. The Ogallala Aquifer was formed between 2 and 6 million years ago, when the Rocky Mountain chain was forming. Because the climate of the Great Plains is arid, water in the aquifer is being used faster than it can be recharged. That's why some scientists refer to using fossil water aquifers as water mining.
Groundwater may also exist on other planets. Images from the Mars Global Surveyor spacecraft show what looked like gullies carved out by rivers of water on the surface of the planet. According to NASA, the water is probably 300 to 1,300 feet (100 to 400 meters) below the surface. Europa, one of Jupiter's moons, may also have subsurface water. As our need for water outweighs the Earth's supply, scientists wonder if we may one day mine for water on the other planets and moons in our solar system.
Water has a lot of unique and amazing properties that make it so important to life. They're why we're constantly looking for better ways to obtain and conserve it.
Plant & Animal Water Consumption
Plants contain even more water than animals do -- most of them are anywhere from 90 to 95 percent water. Just as it does in animals, water regulates the temperature of the plant and transports nutrients through it. But instead of taking in water by drinking and eating, plants get it through dew, irrigation and rainfall.
and transports nutrients through it. But instead of taking in water by drinking and eating, plants get it through dew, irrigation and rainfall.
Plants take in water through their roots, and green ones use it in photosynthesis, which is how they create sugar for food. Plants also need water to support themselves. Pressure from the process of osmosis -- the movement of water from the outside to the inside of the plant's cells -- keeps up the plant's cell walls.
When you water a plant, it sucks up the water through capillary action. Then the water travels from the roots through tubes called xylem vessels. Water reaches the leaves of the plant and escapes through small holes called stomata, which open when the plant needs to cool down. This process is called transpiration and is similar to how people (and some animals) sweat. Carbon dioxide also enters the plant through the stomata.
Processing water is more complicated in animals and people, although it's also similar in a lot of ways. Water that you consume is absorbed in the upper small intestine through osmosis. It enters the bloodstream and is transported all over the body. Unlike plant cells, however, animal cells do not have cell walls. This is why animals have circulatory systems -- otherwise, our cells would absorb water and salt until they swelled. Our circulatory systems move water around our bodies and remove it as needed through sweating and urination.
A few animals, like a microscopic organism called the tart grade, can go without water for an extraordinary period of time. If the tart grade's environment doesn't have enough water, the animal goes into a life without water, called anhydrobiosis. Sugar takes the place of water in its cells, making it impervious to extremes in temperature. Its metabolism lowers, and the tart grade stays at this barely alive state until it has enough water to really live again.
Some plants have also found unique ways to live with little or no water. One way is a variation of photosynthesis called Crassulacean Acid Metabolism (CAM) photosynthesis. In CAM photosynthesis, a plant stores carbon dioxide as acid and keeps its stomata closed during the day to save water (evaporation happens at a slower rate at night). It can even keep its stomata closed at all times if conditions are especially arid. Cacti use CAM photosynthesis to survive the extreme heat and drought of the desert.
Water Properties
The hydrogen bond between water molecules that we talked about in the first section is the reason behind two of water's unique properties: cohesion and adhesion. Cohesion refers to the fact that water sticks to itself very easily. Adhesion means that water also sticks very well to other things, which is why it spreads out in a thin film on certain surfaces, like glass. When water comes into contact with these surfaces, the adhesive forces are stronger than the cohesive forces. Instead of sticking together in a ball, it spreads out.
to the fact that water sticks to itself very easily. Adhesion means that water also sticks very well to other things, which is why it spreads out in a thin film on certain surfaces, like glass. When water comes into contact with these surfaces, the adhesive forces are stronger than the cohesive forces. Instead of sticking together in a ball, it spreads out.
Water also has a high level of surface tension. This means that the molecules on the surface of the water are not surrounded by similar molecules on all sides, so they're being pulled only by cohesion from other molecules deep inside. These molecules cohere to each other strongly but adhere to the other medium weakly. One example of this is the way that water beads up on waxy surfaces, such as leaves or waxed cars. Surface tension makes these water drops round so they cover the smallest possible surface area.
Capillary action is also a result of surface tension. As we mentioned, this happens in plants when they "suck up" water. The water adheres to the inside of the plant's tubes, but the surface tension attempts to flatten it out. This makes the water rise and cohere to itself again, a process that continues until enough water builds up to make gravity begin pulling it back down.
Water's hydrogen bonds are also why its solid form, ice, can float on its liquid form. Ice is less dense than water because water molecules form crystalline structures at freezing (32 degrees Fahrenheit or 0 degrees Celsius) temperatures. The thermal properties of water are also linked to its hydrogen bonds. Water has a very high specific heat capacity, which is the amount of heat per unit mass required to raise its temperature by one degree Celsius. The energy required to raise the temperature of water by one degree Celsius is 4.2 joules per gram. Water also has a high heat of vaporization, which means that it can take a lot of heat without its temperature rising much. This plays a huge part in the climate, because it means that oceans take a long time to warm up.
Water is often known as the universal solvent, which means that many substances dissolve in it. Substances that dissolve in water are hydrophilic. This means that they are as strong or stronger than water's cohesive forces. Salt and sugar are both polar, like water, so they dissolve very well in it. Substances that do not dissolve in water are hydrophobic. This is the source of the saying "oil and water don't mix." Water's solvency is why the water that we use is rarely pure; it usually has several minerals dissolved in it.
The presence of these minerals is the difference between hard water and soft water. Hard water usually contains a lot of calcium and magnesium, but may also contain metals. Soap will not lather well in hard water, but hard water isn't usually dangerous. It can also cause lime scale deposits in pipes, water heaters and toilets.
Some of the latest controversy about water's properties lies in how ice behaves when it melts. Some scientists claim that it looks about the same as it does when it's solid, except that some of its hydrogen bonds are broken. Others claim that it forms an entirely new structure. So for all of its importance, we still don't completely understand water.
How much water is there on Earth?
There's a whole lot of water on Earth! Something like 326,000,000,000,000,000,000 gallons (326 million trillion gallons) of the stuff (roughly 1,260,000,000,000,000,000,000 liters) can be found on our planet. This water is in a constant cycle -- it evaporates from the ocean, travels through the air, rains down on the land and then flows back to the ocean.
liters) can be found on our planet. This water is in a constant cycle -- it evaporates from the ocean, travels through the air, rains down on the land and then flows back to the ocean.
The oceans are huge. About 70 percent of the planet is covered in ocean, and the average depth of the ocean is several thousand feet (about 1,000 meters). Ninety-eight percent of the water on the planet is in the oceans, and therefore is unusable for drinking because of the salt. About 2 percent of the planet's water is fresh, but 1.6 percent of the planet's water is locked up in the polar ice caps and glaciers. Another 0.36 percent is found underground in aquifers and wells. Only about 0.036 percent of the planet's total water supply is found in lakes and rivers. That's still thousands of trillions of gallons, but it's a very small amount compared to all the water available.
The rest of the water on the planet is either floating in the air as clouds and water vapor, or is locked up in plants and animals (your body is 65 percent water, so if you weigh 100 pounds, 65 pounds of you is water!). There's also all the soda pop, milk and orange juice you see at the store and in your refrigerator… There's probably several billion gallons of water sitting on a shelf at any one time!
Why can't we manufacture water?
Water is becoming an increasingly important issue in the developed world. But this issue is nothing new for other, less developed nations. For centuries, clean drinking water has been hard to come by for many populations, especially the poor. In some areas, water may be available, but it's often disease-ridden, and drinking it can be fatal. In other areas, a viable water supply is simply not available at all.
been hard to come by for many populations, especially the poor. In some areas, water may be available, but it's often disease-ridden, and drinking it can be fatal. In other areas, a viable water supply is simply not available at all.
A 2006 United Nations report estimated that as much as 20 percent of the world's population doesn't have access to clean drinking water. This leads us to wonder: If we need it so badly, why can't we just make it?
Water is made of two hydrogen atoms attached to an oxygen atom. This seems like pretty basic chemistry, so why don't we just smash them together and solve the world's water ills? Theoretically, this is possible, but it would be an extremely dangerous process, too.
To create water, oxygen and hydrogen atoms must be present. Mixing them together doesn't help; you're still left with just separate hydrogen and oxygen atoms. The orbits of each atom's electrons must become linked, and to do that we must have a sudden burst of energy to get these shy things to hook up.
Since hydrogen is extremely flammable and oxygen supports combustion, it wouldn't take much to create this force. Pretty much all we need is a spark -- not even a flame -- and boom! We've got water. The hydrogen and oxygen atoms' electrons' orbits have been conjoined.
But we also have an explosion and -- if our experiment was big enough, a deadly one. The ill-fated blimp, the Hindenburg, was filled with hydrogen to keep it afloat. As it approached New Jersey on May 6, 1937, to land after a trans-Atlantic voyage, static electricity (or an act of sabotage, according to some) caused the hydrogen to spark. When mixed with the ambient oxygen in the air, the hydrogen exploded, enveloping the Hindenburg in a ball of fire that completely destroyed the ship within half a minute.
There was, however, also a lot of water created by this explosion.
To create enough drinking water to sustain the global population, a very dangerous and incredibly large-scale process would be required. Still, over a century ago the thought of an internal combustion engine -- with its controlled repeated explosions -- seemed dangerously mad. And as water becomes scarcer, the process of joining hydrogen atoms to oxygen atoms may become more attractive than it is currently. Necessity, after all, is the mother of invention.
But there are safer ways of creating water out of thin air, and projects to do just that are already underway. Read the next page to learn about a few mad scientists who may end up solving the world's impending water crisis.
Creating water from thin air
There's water around us all the time, we just can't see it. The air in our atmosphere contains a varying amount of water vapor, depending on the weather. When it's hot and humid, evaporated water can make up as much as 6 percent of the air we breathe. On cold, dry days it can be as low as .07 percent of the air's makeup [source: U.S. Department of Energy].
evaporated water can make up as much as 6 percent of the air we breathe. On cold, dry days it can be as low as .07 percent of the air's makeup [source: U.S. Department of Energy].
This air is part of the water cycle, an Earth process. Crudely put, water evaporates out of rivers, lakes and the ocean. It's carried up into the atmosphere, where it can collect into clouds (which are actually just accumulations of water vapor). After the clouds reach the saturation point, water droplets will form, which we know as rain. This rain runs off the land and collects into bodies of water, where the whole process begins again.
The problem is, the water cycle goes through dry periods. Because of this, some inventors have begun to wonder, why wait? Why not pull the water vapor right out of the air?
One Australian inventor has done just that. Max Whisson is the creator of the Whisson Windmill, a machine that uses wind power to collect water out of the atmosphere. Whisson points out to the Australian Broadcasting Corporation that water vapor amounts to about "10,000 billion litres [about 2,600 billion gallons] in the bottom kilometere [about .62 miles] of air around the world" [source: ABC]. What's more, this water is replaced every few hours as part of the water cycle.
Whisson's windmill uses refrigerant to cool the blades of his mill, which he's named Max Water. These blades are situated vertically rather than diagonally, so that even the slightest breeze turns them. The cool blades cool the air, causing the water vapor to condense -- become liquid water again. This condensation is then collected and stored. Whisson's windmill can collect as much as 2,600 gallons of water from the air per day.
Whisson says that his biggest challenge isn't the engineering behind his invention but finding the venture capital to back it -- he says that people think it's too good to be true. This problem would sound familiar to a pair of American inventors who have a water-making invention of their own.
Jonathan Wright and David Richards have created a machine that's similar to Whisson's, except that it resembles a collapsible pull-behind camper more than it favors a windmill. This invention -- which its creators call AquaMagic -- pulls air directly from the area surrounding it. Inside the machine, the air is cooled via a refrigerated coil. The air condenses, and the water is collected, purified, and released through a spigot.
The AquaMagic machine -- which currently cost about $28,000 per unit -- can produce up to 120 gallons of purified water in 24 hours, and since it's small it can be toted to disaster sites and Sub-Saharan Africa alike. But it also has one drawback: To produce this much water, AquaMagic requires about 12 gallons of diesel fuel. It's here that the Whisson Windmill (which runs about $43,000 per unit) has a clear advantage over AquaMagic: It's totally green. It runs exclusively on wind power, requiring no fossil fuel. Even the condenser runs off the power generated by the windmill's turbines.
Speaking of the environment, why go to the trouble of collecting water out of the air? Why not simply cause more rain to fall? It may sound far-fetched, but this is actually done -- at times, with catastrophic consequences.
Cloud seeding & the British Disaster
China plans to prevent rain during the opening ceremonies of the 2008 Olympics in Beijing. The process, called cloud seeding, works by firing silver iodide into storm clouds in the days leading up to the event. The Chinese government hoped it could essentially "use up" the existing clouds and assure clear skies for the ceremony.
Chinese government hoped it could essentially "use up" the existing clouds and assure clear skies for the ceremony.
The country's been doing it for decades -- with positive results. But another experiment in cloud seeding, on the other side of the Eurasian land mass, didn't go so smoothly.
Following World War II, the British government was still looking at ways to get a leg up over enemy militaries. The Nazis had come close to destroying Britain, and the United Kingdom had developed a taste for preparation. The British government looked to the skies for an advantage. The Royal Air Force (RAF) began experimenting with cloud seeding. By impregnating the clouds with the particles needed to create a severe thunderstorm, the British could effectively thwart the movement of troops and even literally rain out enemy advances. But the cloud-seeding project went terribly awry.
It's not that the experiments with cloud seeding didn't work. It worked too well.
In 2001, the British Broadcasting Corporation (BBC) investigated rumors that the RAF had seeded the clouds over England. They turned up first-person accounts of some of the pilots who were involved in a top-secret mission called Operation Cumulus. During this August 1952 operation, RAF pilots flew above the cloud line, dropping payloads of dry ice, salt and -- like the Chinese currently use -- silver iodide.
After just 30 minutes, rain began to fall from the infected clouds. At first, the RAF pilots -- dubbed rainmakers by the press -- reputedly celebrated their success. But within the week a deluge began. By the end of the month, North Devon, an area of England near the site of the cloud-seeding experiment, experienced 250 times the normal amount of rainfall.
On August 15, 1952, the day the rain started, an estimated 90 million tons of water coursed through the town of Lynmouth in just one day [source: The Guardian]. Entire trees were uprooted, forming dams and allowing the tide of the two rivers flowing through Lynmouth to grow even stronger in force. Boulders were carried by the current, destroying buildings and carrying residents into the sea. In all, 35 Britons lost their lives that day as a result of the torrential rain. Britain's Ministry of Defense maintains that it had not experimented with cloud seeding prior to the Lynmouth incident.
China and Britain paint two versions of the same picture. On one hand, the Asian nation has successfully created a cloud-seeding program. They've managed to generate irrigation for arid croplands from the ultimate source. But the British disaster shows the potential results of toying with the forces of nature.
And still, we need water more than ever. Using explosions isn't viable to produce water currently, and AquaMagic and Whisson's Windmill aren't being produced on a large enough scale to help with the immediate need for water. Water is a finite resource, and one life on Earth can't do without.
How floods work?
Water is one of the most useful things on Earth. We drink it, bathe in it, clean with it and use it to cook food. Most of the time, it is completely benign. But in large enough quantities, the very same stuff we use to rinse a toothbrush can overturn cars, demolish houses and even kill.
quantities, the very same stuff we use to rinse a toothbrush can overturn cars, demolish houses and even kill.
Flooding has claimed millions of lives in the last hundred years alone, more than any other weather phenomenon. Hurricane Katrina in New Orleans and the 2008 cyclone that struck Myanmar are recent examples of the widespread devastation that flooding can incur.
In this article, we'll find out what makes water change character so rapidly and see what happens when it does. We'll explore the negative impact of floods as well as some of the benefits. We'll also examine how human construction can contain flooding or, in some cases,
Water, water everywhere
To understand how floods work, you have to know something about how water behaves on our planet. The total amount of water on Earth has remained fairly constant for millions of years (though its distribution has varied considerably in that time). Every day, a very small amount of water is lost high in the atmosphere, where intense ultraviolent rays can break a water molecule apart, but new water is also emitted from the inner part of the Earth, by volcanic activity. The amount of water that is created and the amount that is lost are pretty much equal.
of years (though its distribution has varied considerably in that time). Every day, a very small amount of water is lost high in the atmosphere, where intense ultraviolent rays can break a water molecule apart, but new water is also emitted from the inner part of the Earth, by volcanic activity. The amount of water that is created and the amount that is lost are pretty much equal.
At any one time, this volume of water is in many different forms. It can be liquid, as in oceans, rivers and rain; solid, as in the glaciers of the North and South Poles; or gaseous, as in the invisible water vapor in the air. Water changes from state to state as it is moved around the planet by wind currents. Wind currents are generated by the heating activity of the sun. The sun shines more on the area around Earth's equator than it does on areas farther north and south, causing a heat discrepancy over the surface of the globe. In warmer regions, hot air rises up into the atmosphere, pulling cooler air into the vacated space. In cooler regions, cold air sinks, pulling warmer air into the vacated space. The rotation of the Earth breaks this cycle up, so there are several, smaller air-current cycles all along the globe.
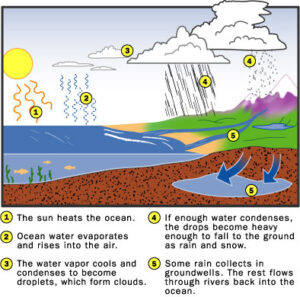 Driven by these air-current cycles, Earth's water supply moves in a cycle of its own. When the sun heats the oceans, liquid water from the ocean's surface evaporates into water vapor in the air. The sun heats this air (water vapor and all) so that it rises through the atmosphere and is carried along by wind currents. As this water vapor rises, it cools down again, condensing into droplets of liquid water (or crystals of solid ice). Collections of these droplets are called clouds. If a cloud moves into a cooler environment, more water may condense onto these droplets. If enough water accumulates in this way, the droplets become heavy enough that they fall through the air as precipitation (rain, snow, sleet or hail). Some of this water collects in large, underground reservoirs, but most of it forms rivers and streams that flow into the oceans, bringing the water back to its starting point.
Driven by these air-current cycles, Earth's water supply moves in a cycle of its own. When the sun heats the oceans, liquid water from the ocean's surface evaporates into water vapor in the air. The sun heats this air (water vapor and all) so that it rises through the atmosphere and is carried along by wind currents. As this water vapor rises, it cools down again, condensing into droplets of liquid water (or crystals of solid ice). Collections of these droplets are called clouds. If a cloud moves into a cooler environment, more water may condense onto these droplets. If enough water accumulates in this way, the droplets become heavy enough that they fall through the air as precipitation (rain, snow, sleet or hail). Some of this water collects in large, underground reservoirs, but most of it forms rivers and streams that flow into the oceans, bringing the water back to its starting point.
Overall, wind currents in the atmosphere are fairly consistent. At any particular time of year, currents tend to move in a certain way across the globe. Consequently, specific locations generally experience the same sort of weather conditions year to year. But on a day-to-day basis, the weather is not so predictable. Wind currents and precipitation are affected by many factors, chiefly geography and neighboring weather conditions. A huge number of factors combine in an infinite variety of ways, producing all sorts of weather. Occasionally, these factors interact in such a way that an atypical volume of liquid water collects in one area. For example, conditions occasionally cause the formation of a hurricane, which dumps a large quantity of rain wherever it goes. If a hurricane lingers over a region, or multiple hurricanes happen to move through the area, the land receives much more precipitation than normal.
Since waterways are formed slowly over time, their size is proportionate to the amount of water that normally accumulates in that area. When there is suddenly a much greater volume of water, the normal waterways overflow, and the water spreads out over the surrounding land. At its most basic level, this is what a flood is -- an anomalous accumulation of water in an area of land.
volume of water, the normal waterways overflow, and the water spreads out over the surrounding land. At its most basic level, this is what a flood is -- an anomalous accumulation of water in an area of land.
A series of storms bringing massive amounts of rain is the most common cause of flooding, but there are others. In the next section, we'll look at some of the ways floods start, as well as some of the factors that determine their magnitude.
Under the weather
 In the last section, we saw that floods occur when an atypical volume of water collects in an area. There are a number of ways this might happen, and there ar
In the last section, we saw that floods occur when an atypical volume of water collects in an area. There are a number of ways this might happen, and there ar
The sort of flooding that most people are familiar with occurs when an unusually large number of rainstorms hit an area in a fairly short period of time. In this case, the rivers and streams that divert the water to the ocean are simply overwhelmed. The varying temperatures of different seasons leads to different weather patterns. In the winter, for example, the air over the ocean might be warmer than the air over the land, causing the wind flow to move from the land out to sea. But in the summer, the air over the land heats up, becoming warmer than the air over the ocean. This causes the wind current to reverse, so that more water from the ocean is picked up and carried over land. This monsoon wind system can cause a period of intense rain that is completely out of step with the climate the rest of the year. In some areas, this flooding may be exacerbated by excess water from melting snow.
 Perhaps the best known example of seasonal flooding is the annual expansion of the Nile River in Egypt. In Ancient Egypt, monsoon rains at the source of the river would cause the waterway to extend out a good distance during the summer. In this case, the flooding was not a disaster, but a godsend. The expanding waters would leave fertile silt all along the banks of the river, making the area ideal farming land once the river had subsided again. This is one of the main factors that allowed civilization to thrive in the Egyptian desert. These days, the river is blocked off by a dam upstream, which collects the summer rain and doles it out throughout the year. This has extended the planting season so that Egyptian farms can grow crops year-round.
Perhaps the best known example of seasonal flooding is the annual expansion of the Nile River in Egypt. In Ancient Egypt, monsoon rains at the source of the river would cause the waterway to extend out a good distance during the summer. In this case, the flooding was not a disaster, but a godsend. The expanding waters would leave fertile silt all along the banks of the river, making the area ideal farming land once the river had subsided again. This is one of the main factors that allowed civilization to thrive in the Egyptian desert. These days, the river is blocked off by a dam upstream, which collects the summer rain and doles it out throughout the year. This has extended the planting season so that Egyptian farms can grow crops year-round.
Another common source of flooding is unusual tidal activity that extends the reach of the ocean farther inland than normal. This might be caused by particular wind patterns that push the ocean water in an unusual direction. It can also be caused by tsunamis, large waves in the ocean triggered by a shift in the Earth's crust.
 Floods may also occur when a man-made dam breaks. We build dams to modify the flow of rivers to suit our own purposes. Basically, the dam collects the river water in a large reservoir so that we can decide when to increase or decrease the river's flow, rather than letting nature decide. Engineers build dams that will stand up to any amount of water that is likely to accumulate. Occasionally, however, more water accumulates than the engineers predicted, and the dam structure breaks under pressure. When this happens, a massive amount of water is released all at once, causing a violent "wall" of water to push across the land. In 1889, such a flood occurred in Johnstown, Pennsylvania. The townspeople were warned that the flood was coming, but many dismissed the alert as unfounded panic. When the rushing wall of water did hit, more than 2,000 people were killed in only a few minutes.
Floods may also occur when a man-made dam breaks. We build dams to modify the flow of rivers to suit our own purposes. Basically, the dam collects the river water in a large reservoir so that we can decide when to increase or decrease the river's flow, rather than letting nature decide. Engineers build dams that will stand up to any amount of water that is likely to accumulate. Occasionally, however, more water accumulates than the engineers predicted, and the dam structure breaks under pressure. When this happens, a massive amount of water is released all at once, causing a violent "wall" of water to push across the land. In 1889, such a flood occurred in Johnstown, Pennsylvania. The townspeople were warned that the flood was coming, but many dismissed the alert as unfounded panic. When the rushing wall of water did hit, more than 2,000 people were killed in only a few minutes.
 The severity of a flood depends not only on the amount of water that accumulates in a period of time, but also on the land's ability to deal with this water. As we've seen, one element of this is the size of rivers and streams in an area. But an equally important factor is the land's absorbency. When it rains, soil acts as a sort of sponge. When the land is saturated -- that is, has soaked up all the water it can -- any more water that accumulates must flow as runoff.
The severity of a flood depends not only on the amount of water that accumulates in a period of time, but also on the land's ability to deal with this water. As we've seen, one element of this is the size of rivers and streams in an area. But an equally important factor is the land's absorbency. When it rains, soil acts as a sort of sponge. When the land is saturated -- that is, has soaked up all the water it can -- any more water that accumulates must flow as runoff.
Some materials become saturated much more quickly than others. To see how this works, just take a bucket of water outside and try wetting various surfaces. Soil in the middle of the forest is an excellent sponge. You could dump several buckets of water on it and it would soak the water right up. Rock is not so absorbent -- it doesn't seem to soak up any water at all. Hard clay falls somewhere in between. Generally, soil that has been tilled for crops is less absorbent than uncultivated land, so farm areas may be more likely to experience flooding than natural areas.
One of the least absorbent surfaces around is concrete. In the next section, we'll see how concrete, asphalt and other human construction can affect flooding.
Take me to the river
 In the last section, we saw that the degree of flooding is determined by the amount of water that accumulates in an area, as well as the nature of the land surface. As civilization has expanded, human beings have altered the landscape in a number of ways. In the Western World, one of the most significant changes has been covering the ground in asphalt and concrete. Obviously, these surfaces are not the best sponges around: Almost all rain that accumulates becomes runoff. In an industrialized area without a good drainage system, it may not take much rain to cause significant flooding.
In the last section, we saw that the degree of flooding is determined by the amount of water that accumulates in an area, as well as the nature of the land surface. As civilization has expanded, human beings have altered the landscape in a number of ways. In the Western World, one of the most significant changes has been covering the ground in asphalt and concrete. Obviously, these surfaces are not the best sponges around: Almost all rain that accumulates becomes runoff. In an industrialized area without a good drainage system, it may not take much rain to cause significant flooding.
Some cities, such as Los Angeles, have constructed concrete flood-relief channels to prevent this problem. When it rains a lot, the water flows into these channels, which meander out of the city where the water can be better absorbed. These sorts of systems may cause flooding farther down the line, however. When you cover an area in concrete and asphalt, you are essentially cutting off part of the Earth's natural sponge, so the rest of the sponge has a lot more water to deal with.
A similar problem can arise with levees, large walls built along rivers to keep them from overflowing. These structures extend the natural banks of the river so that much more water can flow through it. But while they may be effective at keeping water out of one area, they usually make problems worse for an area down the line, where there are no levees. That area gets all the flood waters that would have spread out farther up river. Another danger of levees is that, like dams, they can break. When this happens, a large amount of water flows out onto the land in a short period of time. This can cause some of the most dangerous flood conditions.
 People haven't had much success with controlling flooding along coastlines. Excessive water in these areas is particularly destructive to man-made structures because of the erosion it causes. One method of controlling this erosion is to build fences and walls where the water meets the land. This keeps the power of the waves at bay, so they don't wear down the beach. But the structures also interfere with the process of beach formation. When you block the water from moving against the coast, the ocean can't distribute sand and you don't get beautiful beaches.
People haven't had much success with controlling flooding along coastlines. Excessive water in these areas is particularly destructive to man-made structures because of the erosion it causes. One method of controlling this erosion is to build fences and walls where the water meets the land. This keeps the power of the waves at bay, so they don't wear down the beach. But the structures also interfere with the process of beach formation. When you block the water from moving against the coast, the ocean can't distribute sand and you don't get beautiful beaches.
Another problem with fences and walls is that there is only so much they can do. Fundamentally, beaches are changing environments, molded by the overpowering force of the ocean. They are, by their very nature, supposed to be eroded and moved by the dynamic action of waves. Flooding is a regular part of this process, and most likely will continue to be no matter what we do.
 The same can be said for many inland areas. While a river may appear to us to be a stable, unmovable feature of the landscape, it is really a vibrant, dynamic entity. This is particularly true of big rivers, such as the Mississippi in the United States and the Yangtze and Huang He in China. Over time, these waterways expand, shift their path dramatically and may even change the direction of flow. For this reason, the land around the banks of a river is highly susceptible to flooding.
The same can be said for many inland areas. While a river may appear to us to be a stable, unmovable feature of the landscape, it is really a vibrant, dynamic entity. This is particularly true of big rivers, such as the Mississippi in the United States and the Yangtze and Huang He in China. Over time, these waterways expand, shift their path dramatically and may even change the direction of flow. For this reason, the land around the banks of a river is highly susceptible to flooding.
Unfortunately, rivers are also natural draws for civilization. Among other things, they provide a constant supply of water, rich soils and an easy means for transportation. When the water level is low, people build all along its banks and enjoy all its benefits. At some point, it comes time for the water to shift, and the people who have built along the flood plains quickly discover that they are living on unsound ground. If there is extensive construction in these
Come hell or high water
 The worst damage from floods, the loss of life and homes, is caused primarily by the sheer force of flowing water. In a flood, two feet (61 cm) of water can move with enough force to wash a car away, and 6 inches (15 cm) of water can knock you off your feet. It may seem surprising that water, even a lot of water, can pack such a wallop. After all, you can peacefully swim in the ocean without being knocked around, and that's a massive amount of moving water. And in most cases, a flowing river isn't strong enough to knock you over. So why do flood waters behave differently?
The worst damage from floods, the loss of life and homes, is caused primarily by the sheer force of flowing water. In a flood, two feet (61 cm) of water can move with enough force to wash a car away, and 6 inches (15 cm) of water can knock you off your feet. It may seem surprising that water, even a lot of water, can pack such a wallop. After all, you can peacefully swim in the ocean without being knocked around, and that's a massive amount of moving water. And in most cases, a flowing river isn't strong enough to knock you over. So why do flood waters behave differently?
 Flood waters are more dangerous because they can apply much more pressure than an ordinary river or a calm sea. This is due to the massive differences in water volume that exist during many floods. In a flood, a lot of water may collect in an area while there is hardly any water in another area. Water is fairly heavy, so it moves very quickly to "find its own level." The bigger the difference between water volumes across an area, the greater the force of movement. But at a particular point, the water doesn't look so deep, and so doesn't seem particularly dangerous -- until it's too late. Nearly half of all flood deaths result from people attempting to drive their cars through rushing water. There is much more water in the ocean than in a flood, but it doesn't knock us over because it is fairly evenly distributed -- water in a calm sea isn't rushing to find its own level.
Flood waters are more dangerous because they can apply much more pressure than an ordinary river or a calm sea. This is due to the massive differences in water volume that exist during many floods. In a flood, a lot of water may collect in an area while there is hardly any water in another area. Water is fairly heavy, so it moves very quickly to "find its own level." The bigger the difference between water volumes across an area, the greater the force of movement. But at a particular point, the water doesn't look so deep, and so doesn't seem particularly dangerous -- until it's too late. Nearly half of all flood deaths result from people attempting to drive their cars through rushing water. There is much more water in the ocean than in a flood, but it doesn't knock us over because it is fairly evenly distributed -- water in a calm sea isn't rushing to find its own level.
The most dangerous floods are flash floods, which are caused by a sudden, intense accumulation of water. Flash floods hit an area soon after water begins to accumulate (whether from excessive rain or another cause), so a lot of the time, people don't see them coming. Since there is a great deal of water collected in one area, flash-flood waters tend to move with a great deal of force, knocking people, cars and even houses out of the way. Flash floods can be particularly devastating when a heavy thunderstorm dumps a high volume of rain on a mountain. The water moves down the mountain at tremendous speed, plowing through anything in the valleys below.
 One of the worst flash floods in U.S. history occurred in 1976, in Big Thompson Canyon, Colorado. In less than five hours, thunderstorms in nearby areas dumped more rain than the region ordinarily experiences in a year. The Big Thompson River, normally a shallow, slow-moving waterway, abruptly transformed into an unstoppable torrent, dumping 233,000 gallons (882,000 L) of water into the canyon every second. Thousands of campers had gathered in the canyon to celebrate the centennial of the state of Colorado. The flood happened so quickly that there was no time to issue a warning. When it hit, hundreds of people were injured, and 139 were killed.
One of the worst flash floods in U.S. history occurred in 1976, in Big Thompson Canyon, Colorado. In less than five hours, thunderstorms in nearby areas dumped more rain than the region ordinarily experiences in a year. The Big Thompson River, normally a shallow, slow-moving waterway, abruptly transformed into an unstoppable torrent, dumping 233,000 gallons (882,000 L) of water into the canyon every second. Thousands of campers had gathered in the canyon to celebrate the centennial of the state of Colorado. The flood happened so quickly that there was no time to issue a warning. When it hit, hundreds of people were injured, and 139 were killed.
A less catastrophic sort of damage is simple dampness. Most buildings can keep out the rain, but they aren't built to be water-tight. If the water level is high enough, loads of water seeps into houses, soaking everything. But in most cases, the major damaging element is not the water itself, but the mud it brings with it. As water flows over the landscape, it picks up a lot of junk. When the flood is over, the water level drops and everything eventually dries out, but the mud and debris stick around.
 In 1966, a major storm flooded the Arno, an Italian river that runs through the city of Florence. The small city, one of the art capitals of the world, was overrun with water, mud and general slime. In addition to the loss of life and the damage to buildings, there was a great deal of damage to the city's art collection. Mud and slime covered almost everything stored in the city's basements and ground-level rooms. Through many years of work, scientists and art historians have been able to restore most of the damaged artifacts to good condition.
In 1966, a major storm flooded the Arno, an Italian river that runs through the city of Florence. The small city, one of the art capitals of the world, was overrun with water, mud and general slime. In addition to the loss of life and the damage to buildings, there was a great deal of damage to the city's art collection. Mud and slime covered almost everything stored in the city's basements and ground-level rooms. Through many years of work, scientists and art historians have been able to restore most of the damaged artifacts to good condition.
Another sort of flood damage is the spread of disease. As water flows over an area, it can pick up all sorts of chemicals and waste products, leading to extremely unsanitary conditions. Essentially, everything and everyone in a flood is floating along in one big soup. While diseases usually aren't created by these conditions, they are more easily transferred (most diseases spread through water more readily than they move through the air). If you are in a flooded area, it is very important that you drink only bottled or boiled water and observe other sanitation guidelines. To learn more about what to do in flooded conditions, check out this guide put out by the Center for Disease Control.
We'll never be able to stop flooding. It is an unavoidable element in the complex weather system of our atmosphere. We can, however, work to minimize the damage inflicted by flooding, by building sophisticated dams, levees and canal systems. But the best way to avoid flood damage may be to back out of flood-prone areas altogether. As with many natural phenomena, the most sensible reaction to flooding may be to get out of the way
Source: http://science.howstuffworks.com

 ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state (water vapor or steam). Water also exists in a liquid crystal state near hydrophilic surfaces. Under nomenclature used to name chemical compounds, Dihydrogen monoxide is the scientific name for water, though it is almost never used.
ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state (water vapor or steam). Water also exists in a liquid crystal state near hydrophilic surfaces. Under nomenclature used to name chemical compounds, Dihydrogen monoxide is the scientific name for water, though it is almost never used.
